Blue Salt Mine, Klodawa, Poland
History
Kłodawa [kwɔˈdava] is a town in central Poland with 6,699 inhabitants (2014). It is situated in the Greater Poland Voivodeship (since 1999), having previously been in Konin Voivodeship (1975–1998). Kłodawa lies on the Rgilewka (a tributary of the Warta River). The town contains the largest operating salt mine in Poland, extracting halite and salts of potassium and magnesium.
In 1939 Polish professor Edward Walery Janczewski discovered geological layer of salt between Izbica Kujawska and Solca Wielka. It was 63 kilometers long and 4 kilometers wide. First drillings were done during German occupation of Poland and it was realized that Kłodawa is the best place to build a salt mine; it was also said that Izbica Kujawska had a good salt supply, but much smaller than Kłodawa.

The first two mineshafts (called Michał and Barbara, – named after the first director of the mine and Saint Barbara), were built from 1950 to 1954. The salt has been drilled since 1956. Until 1966 horses were the main way to drill the salt, in this year the electronic traction drive was built. The third mineshaft, located about 4 kilometers from Michał and Barbara, is named Chrobry (after first Polish king, Bolesław I Chrobry).
There is an underground touristic route in the mine, opened for visitors. It is scheduled to open the first underground sanatorium in Poland in Kłodawa Salt Mine. On September 7, 2012 the salt mine suffered from a fire.
Located in central Poland, the Kłodawa salt dome is 26 km long and about 2 km wide. Exploitation of the dome started in 1956, currently rock salt extraction is carried out in 7 mining fields and the 12 mining levels at the depth from 322 to 625 meters below sea level (m.b.s.l.). It is planned to maintain the mining activity till 2052 and extend rock salt extraction to deeper levels. The dome is characterized by complex geological structure resulted from halo kinetic and tectonic processes.
Inclusions in anhydrite crystals from blue halite veins in the Kłodawa Salt Dome
(Zechstein, Poland)
The occurrence of both the blue and violet halites is one of the most interesting phenomena in nature. Despite numerous laboratory and field works, their origin in natural evaporitic environments has not been satisfactorily explained. In the Kłodawa Salt Dome (Zechstein, Central Poland), blue or violet halites occur relatively frequently. Their accumulations differ in size and intensity of colors. In this paper, petrological features of anhydrite crystals derived from one of the largest outcrops of the blue halite at the Kłodawa Salt Mine are presented. Anhydrite is one of solid inclusions encountered in blue-colored halite crystals. Special attention was paid to fluid inclusions present in this anhydrite. The micro thermometric measurements showed two directions of homogenization, i.e., towards the liquid phase (LG→L, LL→L) or towards the gas phase (LG→G). In the former case, the temperatures ranged from 174 to 513°C, whereas in the latter one, the values from 224 to 385°C were measured. The composition of inclusions is relatively variable. We can observe transparent and opaque daughter minerals as well as CO2 in the liquid phase accompanied by a variable amount of methane or hydrogen sulphide. These features of inclusions indicate that anhydrite crystals and, thus, blue halite were formed under the influence of hydrothermal conditions. Observations in the mine workings combined with petrological studies enable to conclude that blue coloration of halite crystals is controlled by three factors: a high temperature, reducing conditions and defects in halite lattice related to tectonic stress.

INTRODUCTION
The blue or violet halite crystals are very rare in nature although they were noticed in many salt formations of various ages. They were found, inter alia, in Cambrian KCl-deposits from the southern part of the Siberian platform (Pustyl’nikov, 1975), Mississippian rocks of eastern Canada
(Roulston and Waugh, 1983; Waugh and Urquhart, 1983; Davison, 2009), Lower Permian of the Solikamsk Depression in the Ural Foredeep (Vinokurov, 1958; Ivanov and Voronova, 1972; Smetannikov, 2011), within the Verkhnepechora Basin (Ivanov and Voronova, 1968) as well as in the Kramatorsk Series of Donbass (Bobrov et al., 1968), Lower Permian of the Delaware Basin, south-eastern New Mexico, USA (Bickham, 2012), Upper Permian (Zechstein) salt deposits of Germany (Borchert, 1959), Cretaceous to Paleogene (Maha Sarakham Formation) of the Khorat Plateau, north-eastern Thailand (Suwanich, 1983, Tabakh et al., 1999), Paleogene and Neogene of Northern and Central Iran (Rahimpour-Bonab and Alijani, 2003; Baikpour et al., 2010), and Miocene of the Carpathian Foredeep in Ukraine (Koriń, 1994).

The nature of such coloration has been investigated for over 150 years by many researchers representing various fields. One of the first scientists who undertook in-depth analysis of blue halite was Kreutz (1892). He analyzed the results of experiments and observations made by previous authors, and completed a series of his own experiments on natural and synthetic halite crystals. Based on these data he concluded that: 1/ blue colour does not fade when halite crystals are heated in oxygen-free atmosphere, but disappears under “flame of oxidation”; 2/ previously discolored and naturally colorless halite crystals become colored when heated in potassium or sodium vapors, although not all originally colorless crystals become colored; and 3/ under the same experimental conditions, synthetic halite crystals are not colored.
These and other observations and experiments enabled Kreutz (1892) to present the theory that the color of halite crystals originated from very small admixtures of iron or other metals, which were below the detection limits of analytical methods used at that time.
The development of modern analytical methods since the late 19th century has provided new valuable data. The new studies (see Sonnenfeld 1995) focused mostly on laboratory analyses of blue halite properties and on the methods of its coloration, and they gave rise to new hypotheses on the origin of color in natural halite. It was found that, in addition to the above-mentioned factors, coloration of halite can be obtained by crystallization from NaCl- and KCl-saturated solutions containing 320 g/l MgCl2, by electric spark puncture and by the action of ionizing UV, α, β and γ radiations. Moreover, it was also observed that the physico-chemical properties of artificially colored halite crystals differ from the natural ones. A large number of papers concerning halite crystals coloration were reviewed and discussed by Sonnenfeld (1995). He concluded that most of experimental results were obtained under conditions which do not occur in the nature. He has also questioned the widely accepted view that the color of natural halite results from radiation caused by radioactive decay of 40K isotope.
In recent years, detailed observations and studies on the occurrence of blue halite were carried on at the Kłodawa Salt Dome (Natkaniec-Nowak and Toboła, 2003; Toboła et al., 2007; Janiów et al., 2008). As a result of this research, 19 key exposures were described together with their relations to the surrounding rocks and mineral composition, as well as with visual estimation of the intensity, variability, hue and saturation of color. Standard microscopic examinations of thin sections of blue halite revealed the presence of birefringent areas in the crystals. Unfortunately, their correlation with colored areas was difficult because halite colors are invisible in thin sections (Heflik et al. 2008).
Similarly, in thick sections, in which blue color is clearly visible under transmitted light, characteristic birefringence does not coincide with colored areas, but both features coexist side by side. Moreover, observations of thick sections revealed also the common presence of intergrowths, both transparent and opaque. The SEM-EDS examinations showed that transparent intergrowths are the mixtures of sodium, magnesium and potassium chlorine compounds, whereas opaque intergrowths are iron sulphides, although their crystal habits do not correspond to well-known and most common minerals, such as pyrite or marcasite (Toboła and Natkaniec-Nowak, 2008). The fluid inclusion studies demonstrated the presence of several inclusion types differing in geometric patterns. The inclusions are filled with highly concentrated brines as well as with gases (CO2, CO, COS, O2, N2, CH4, C3H8), with aromatic hydrocarbons and with liquid, long-chained hydrocarbons (Toboła and Wesełucha-Birczyńska, 2008; Wesełucha-Birczyńska et al., 2008). Structural studies of halite samples with varying degrees of staining showed deformation of the symmetry of regular structure (Zelek et al., 2007, 2014; Stadnicka and Zelek, 2008). All these data clearly demonstrated the complexity of processes leading to the formation of colored varieties of halite in the salt dome.
The aim of present studies is to estimate the formation temperature of the blue salts. Previous investigations carried out for blue halite crystals suggested in some cases high temperatures, up to 347°C (Toboła and Wesełucha-Birczyńska, 2008). However, these data should be treated with caution because of some halite properties such as easy recrystallization and reaction with solutions contained in inclusions (e.g., Roedder, 1984b). Hence, more precise and credible data can be obtained for minerals coexisting with the blue halite. Anhydrite, accompanied by iron sulphides (pyrite) and quartz, is the most common mineral. Such paragenesis as well as the fluid inclusions assemblage (FIA) in anhydrite crystals document atypical conditions of the blue halite formation and the thermal impact on salt rocks.
GEOLOGICAL SETTING
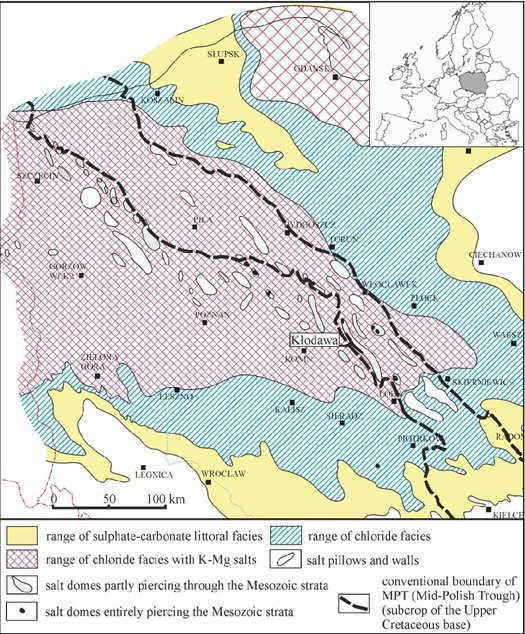
The Kłodawa Salt Dome is located in the Kujawy region (Central Poland). This region is characterized by the most complete lithostratigraphic succession and the maximum thickness of the Zechstein salt-bearing formation. Detailed data concerning the relationships of salt-bearing formation to the surrounding rocks, its tectonics, lithostratigraphy and facies changes can be found in a number of publications (see e.g., Dadlez, 2003; Dadlez et al., 1995; Krzywiec, 2004, 2006). The Kłodawa Salt Dome belongs to the Izbica Kujawska-Łęczyca salt ridge (Fig. 1), which is considered as the largest salt diapir in Poland. Studies on the diapir structure indicated its strong NW-SE elongation. The length of the diapir is about 26 km, and its width varies from 0.5 to 2 km (e.g., Werner et al. 1960; Tarka, 1992; Burliga, 2014; Burliga et al., 1995). The geological cross-sections show the asymmetrical shape of the diapir: its NE slope dips moderately eastward at 55-70o, but its SW slope is almost vertical. The diapir is enclosed within deformed Mesozoic (Triassic-Jurassic) and, partly, Neogene deposits, and is covered by Quaternary and Neogene sediments. The Kłodawa salt deposit is built mainly of rocks representing fully developed Zechstein PZ2-PZ4 cyclothems. These are claystones, dolomites, anhydrites, rock salts and K-Mg salts. The lowermost Zechstein cyclothem PZ1 is known only as tectonically transported blocks (Charysz, 1973; Burliga et al., 1995).
The internal structure of the Kłodawa Salt Dome is complicated due to deformation of salt series. The main effect of salt movement during the diapir rising is the piercing of the Older Halite (Na2) by the Younger (Na3) and the Youngest (Na4) halites, as well as the occurrence of tectonic pinch-outs and high-amplitude narrow folds. Such complicated deformation resulted from different rheological properties of rocks. In general, two NW-SE elongated anticlines predominate in the SW and NE parts of the diapir (Fig. 2). These are separated by a deep central syncline built of the youngest salt layers (Charysz, 1973, Tarka, 1992; Burliga, 1994).
An important role in the internal structure of the salt dome is played by epigenetic salts. Their presence is closely associated with salt deformation processes that facilitated migration of solutions. In the Kłodawa Salt Dome the epigenetic salts form veins and accumulations of variable size. Their mineral composition includes large halite crystals as well as polyhalite, carnallite and sylvite (Stańczyk, 1970; Stasik-Stańczak, 1976).
OCCURRENCE OF THE BLUE HALITE IN THE KŁODAWA SALT DOME
The blue halite is relatively common at the Kłodawa Mine. It was found at all main mining levels and in many interlevel workings, in various rocks of the PZ2, PZ3 and PZ4 cyclothems (Natkaniec-Nowak and Toboła, 2003; Toboła et al., 2007; Janiów et al., 2008). The blue halite forms very diverse concentrations with respect to its size and color, its contact with the surrounding rocks, and the occurrence of accompanying minerals.

The halite usually forms very small accumulations (from a few to several centimetres across) hosted in small veins or irregular accumulations of recrystallized salt. Salt crystals sometimes contain intergrowths of sylvite, carnallite and polyhalite . The blue color appears as point clusters or small streaks within single halite crystals. Such accumulations are most common within the Older Halite (Na2) or Younger Halite (Na3).

 The larger accumulations of blue-colored halite crystals reach from a few to, occasionally, several tens of meters in length. Their outcrops were encountered at mining levels 525, 562, 600 and 750.
The larger accumulations of blue-colored halite crystals reach from a few to, occasionally, several tens of meters in length. Their outcrops were encountered at mining levels 525, 562, 600 and 750.
At the level 525, a continuous zone of blue halite was recognized within the Older Halite (Na2), over 50 m long and from 4 to 15 m wide. It extends from the KS29-KS21 chambers through the area of KSc18 and KSc12 cylindrical chambers, and continues towards the KS16a chamber as well as galleries leading to the above-mentioned workings.

A) Small clusters and streaks of blue color within halite crystal accompanied by carnallite and polyhalite (mining level 600 m, chamber KS1d). B) Larger, irregular concentration of blue halite within striped salt (Older Halite) (mining level 525 m, chamber KSc14). C) Coarse-crystalline halite with small intergrowths of sylvite (marked by arrows) with blue rim (mining level 630 m, gallery between chambers KS16b and KS17b). D) Streaks and elongated concentration of blue halite in white halite and sylvite (mining level 562 m, ventilation gallery between chambers KS38 and KS39). E) Blocks of primary striped salt (Older Halite) with blue rims embedded within white epigenetic vein of halite and sylvite (mining level 600 m, roof of chamber KS39). F) Large blue-colored halite crystals inside a vein of white carnallite with admixture of sylvite (mining level 750 m, crossing of galleries GPT2 and GPT2a).A characteristic feature of this zone is the occurrence of both epigenetic structureless veins and nests of large halite crystals, and rock salt aggregates with preserved residual ”striped” structure (Toboła et al., 2007; Janiów et al., 2008).
In the halite crystals, blue coloration shows various patterns. The most common features of the “striped” halites are irregular, spotty accumulations with fuzzy contours and varying degrees of blue color saturation. In the structureless salt aggregates, where halite crystals occasionally exceed 20cm in diameter, blue color is much rarer. It can be observed only in some halite crystals as single spots or spot clusters, up to several millimeters across. Blue coloration is often associated also with transparent solid inclusions varying in size from 0.5 to 2-3 mm, embedded within halite crystals which show thin blue rims.
One of the largest accumulations of the colorful halite, extending over a distance of 12 meters, is located at the mining level 562, in the ventilation gallery between chambers KS38 and KS39, within the Older Halite (Na2). The main mass comprises transparent or white halite and sylvite in various proportions. The colored halite forms strongly elongated streaks of diffused boundaries, up to several centimeters thick, extending continuously through the enclosing white salts. The color of these streaks changes from light blue through bluish-violet to dark navy blue. Locally, even purple or yellow halite crystals can be found (Toboła et al., 2007; Janiów et al., 2008).
At the mining level 600 in the end part of chamber KS39, there is an extension of the above-mentioned outcrop of blue halite. However, in contrast to the accumulation at the level 562, the greatest amounts of blue salt occur at the contact of striped primary salts (Na2) and epigenetic sylvinite. Streaks or individual small light-blue halite crystals are observed much more rarely. The most intense blue color appears along the edges of tectonic blocks of striped halite (Na2) embedded within sylvinite veins (Fig. 3E).
Other major exposures at this mining level were found in the galleries NW I and NE VII (Toboła et al., 2007; Janiów et al., 2008). They are hosted inside laminated salt of the Younger Halite (Na3). Similarly to the above-mentioned occurrences in the ventilation gallery and chamber KS39, the matrix of the vein consists of white epigenetic sylvinite, within which the blue halite forms blurry streaks, up to about 20-30 cm thick and generally parallel to the course of that epigenetic vein. The largest concentrations of halite showing the highest intensity of blue color are located at the contact of the vein and the Younger Halite (Na3).
Another variety of large halite accumulation is located at the mining level 750 (Toboła et al., 2007; Janiów et al., 2008) within the Younger Potash Salts (K3). The wall-rocks of the vein are composed of epigenetic carnallite with an admixture of sylvite, in which single crystals of light-blue halite are embedded (Fig. 3F). The colored halite is generally scattered in the vein as single crystals or small aggregates. Larger accumulations in the form of streaks of darker-blue halite are observed at the contact of the vein with K-Mg salts (K3), especially where these salts are accompanied by the rock salt.
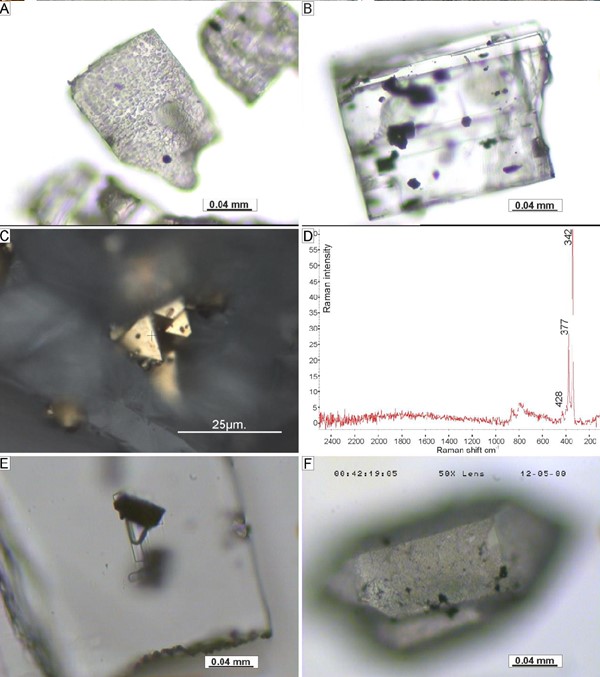
Microscopic images of: A) Rough surface of anhydrite crystals. B) Intergrowths of opaque minerals within anhydrite crystals (transmitted light). C) Euhedral pyrite intergrowths in anhydrite crystal (reflected light). D) Raman spectra of pyrite intergrowth in C. E) Solid intergrowths associated with two-phase fluid inclusion (transmitted light). F) Euhedral quartz crystal with rough surface (transmitted light).
CONCLUSIONS
The origin of blue or violet halite crystals in natural environments has not been adequately explained so far. Numerous laboratory experiments using various methods of halite coloration do not reflect the natural conditions (Sonnenfeld, 1995). The most common opinion that radioactive decay of potassium 40K isotope is responsible for halite coloration does not explain adequately the accumulation of blue halite in the salt formations because, up to now, blue halite has not been found in the primary potassium salt beds.
Blue salt mine around the world:

Iran, Semnan Blue Salt (edible)– 60-80 Mt Annually – Edible blue salt – Salt lamp |

Iran, Garmsar blue salt– Limited amount – Salt lamp |

Poland, Kłodawa blue Salt– Limited amount – Salt lamp |

USA, New Mexico blue salt Limited amount– Limited amount – Salt lamp |
Studies on anhydrite intergrowths in blue halite from one of the largest underground exposures in the Kłodawa Salt Dome indicate that blue coloration of halite is a combined effect of at least three factors: 1) high temperatures (above 250°C) resulting from migration of hydrothermal solutions within the salt formation, 2) reducing conditions, as demonstrated by the occurrence of sulphide minerals, 3) defects of crystal structure, caused by the tectonic activity and, especially, by the stress developed in the shear zone.


The origin of hydrothermal solutions is related to the basement of Zechstein salt formations and to the development of salt diapirs (cf. Wagner and Burliga, 2014). Their migration through the salt formation caused the evolution of their chemical composition. First of all, the solutions became enriched in some ions: K, Mg, Ca, Na, Cl and SO4 due to dissolution of the lower part of the Zechstein salt formations. The processes of dissolution and recrystallization facilitated the tectonic movements and the development of the salt diapir but they also resulted in changes of mineral composition of beds within the salt dome and in the appearance of minerals which are not typical of marine evaporites.
Acknowledgements: The investigations were supported by the AGH University of Science and Technology in Kraków, grant No. 11.11.140.320. Helpful comments by the referees, G. Czapowski and an anonymous one, are appreciated.












Comments (2)
Like!! Really appreciate you sharing this blog post.Really thank you! Keep writing.
I am genuinely delighted to read this blog posts which carries lots of valuable data, thanks for providing such data.
Also visit my homepage: https://royalcbd.com/